| 50. |
The contact angle of the colloidal liquid-gas interface and a hard wall P. P. F. Wessels, M. Schmidt, and H. Löwen, J. Phys.: Condens. Matt. 16, S4169 (2004). Locate in [bare] [illustrated] list. Get [full paper] as pdf. Extract. We consider the Asakura-Oosawa-Vrij model in contact with a planar hard wall at liquid-gas coexistence. Using density functional theory, the liquid-gas, wall-liquid and wall-gas interfacial free energies are calculated and inserted into Young's equation to obtain the contact angle between the liquid-gas interface and the wall. The relations to previous work are discussed in detail. [more] 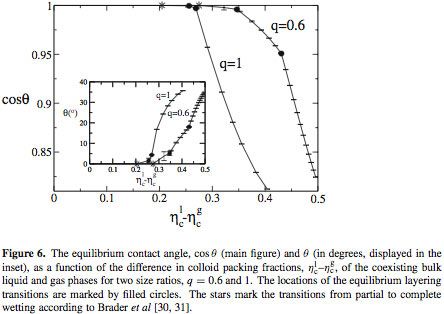 Read the [full paper] as pdf. |
Colloid-polymer mixtures: Beyond the AOV model
Extensions include taking into account an explicit solvent of point particles [27], penetrability of (small) colloids into polymers [28], colloid-induced polymer compression [31], the influence of polymer interactions on fluid-demixing [34] and on the contact angle of the colloidal liquid-gas interface and a hard wall [50], as well as the stability of the floating liquid phase in sedimenting colloid-polymer mixtures for non-ideal polymers [52].Fluid interfaces
Colloid-polymer mixtures display fluid-fluid interfaces [21] [44], relevant for laser-induced condensation [35], capillary condensation [43] and evaporation [48], immersion in porous media [41], the appearance of the floating liquid phase [52], the competition between sedimentation and phase coexistence [51], tension at a substrate [45], the experimental observation of thermal capillary waves [47], and the contact angle of the liquid-gas interface and a wall [50]. In colloidal rod-sphere mixtures fluid-fluid interfaces were investigated with theory [30] and simulation [42]. Hard sphere fluids were considered at surfaces of porous media [37], in random fiber networks [39], and in one dimensional cases [46].[more]